Optive® OMEGA™ UD
OPTIVE OMEGA™ UD is a unique combination formula containing linseed
oil, a source of Omega-3, for rapid and long-lasting
relief of lipid-deficient dry eye symptoms.1,2
OPTIVE OMEGA™ UD helps protect against tear evaporation by providing
lubrication and moisture to the eye surface, supporting
tear film stability.1
OPTIVE OMEGA™ UD supports hydration and helps maintain healthy ocular
surface cells by restoring osmotic balance and
protecting natural tears through lipid enhancement.1,3
Preservative-free, convenient single-use vials.
No shaking required prior to use.4
Innovative technology
Introducing innovative technology by combining linseed oil, a source
of Omega-3, CMC, and osmoprotectants into one
drop.
OPTIVE OMEGA™ UD provides rapid and long-lasting relief of symptoms
associated with lipid-deficient Dry Eye Disease.1,2
Castor and Linseed oils: 3-5
Stabilizes the lipid layer
Protects natural tears
Anti-inflammatory effect in DED
Carboxymethylcellulose (CMC): 6-8
Improved comfort
Effective lubrication
Stays on the eye longer
Osmoprotectants 9-11
Provides hydration
Protects against hyperosmotic stress
Restores osmotic balance
Instillation
To apply the eye drops, first wash your hands, then twist the top off the unit dose vial. Place 1 or 2 drops of OPTIVE OMEGA™ UD in the affected eye(s) as needed.
Ingredients
OPTIVE OMEGA™ UD is supplied in foil wrapped pouches. Each vial contains 0,4 ml of solution.
Precautions
Add-on treatment
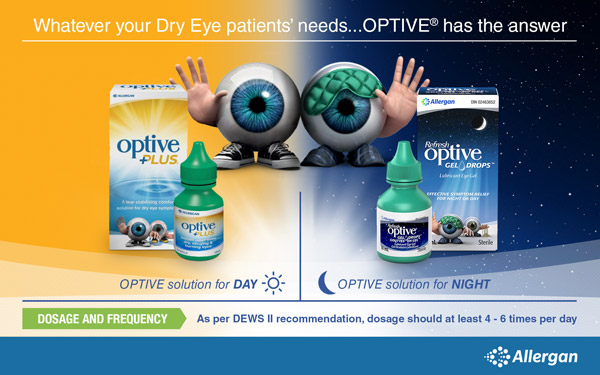
Higher-viscosity OPTIVE® GEL DROPS are an effective add-on treatment to OPTIVE PLUS™ , providing long-lasting relief both during the day and at night.
OPTIVE OMEGA™ Unit Dose Eye Drops: Contains
carboxymethylcellulose sodium 5 mg/ml, glycerine 10 mg/ml,
polysorbate 80 5 mg/ml, linseed oil, and castor oil.
OPTIVE PLUS™ Eye Drops: Contains
carboxymethylcellulose sodium 5 mg/ml, glycerine 10 mg/ml, castor
oil 2,5 mg/ml, and polysorbate 80 5 mg/ml.
OPTIVE FUSION™ Eye Drops: Contains sodium
hyaluronate 1 mg/ml, carboxymethylcellulose sodium 5 mg/ml, and
glycerine 9 mg/ml.
OPTIVE® Lubricant Eye Gel Drops: Contains
carboxymethylcellulose sodium 10 mg/ml and glycerine 9 mg/ml.
For full prescribing information, refer to the
Instructions for Use.
Allergan Pharmaceuticals (Pty) Ltd, PO Box 6024, Halfway House,
1685, South Africa (Co. Reg. no. 1984/005576/07)
Telephone: +27 (0) 11 545 6600, Facsimile: +27 (0) 11 315 6008
www.allergan.co.za
© 2021. ®/™ Registered Trademark/Trademark of Allergan, Inc. Date
of preparation: June 2021. SA-OPT-2150062
Clinical References
1. OPTIVE OMEGA™ UD DFU.
2. Fogt JS, et al. Clin Ophthalmol 2019;13:2553-2561.
3. Downie LE, et al. Ocul Surf 2020;18:148-157
4. Beard BJ, et al. Abstract 115550, Presented at Am Acad Omptometry. 2011
5. Rashid S, et al. Arch Ophthalmol 2008;126(2):219-225
6. White CJ, et al. Contact Lens & Anterior Eye 2014;37:81-91.
7. Garret Q, et al. Invest Ophthalmol Vis Sci 2007;48:1559-1567.
8. Monaco G, et al. Eur J Ophthalmol 2011;21(3):243-250.
9. Foulks GN. Drugs Aging 2008;25(2):105-118.
10. Mager WH, Siderius M. FEMS Yeast Research 2002;2:251-257
11. Yancey PH. J Exp Biol 2005;208:2819-2830





